
COVID deal? CR?
Look past speeches. The answers?
It’s all in the ayes.
Federal Updates
House passes Anti-Asian Discrimination Resolution
The House approved a resolution introduced by Rep. Grace Meng (D-NY) that denounces anti-Asian sentiment and actions related to the COVID-19 public health emergency. The resolution (H.Res. 908) calls on all public officials to condemn and denounce anti-Asian sentiment, racism, discrimination, and religious intolerance related to COVID-19 and for federal law enforcement officials, working with state and local officials, to “investigate and document all credible reports of hate crimes and incidents and threats” directed at Asian Americans and those of Asian descent.
While anti-discrimination orders and resolutions related to the treatment of Asian Americans during COVID-19 have been adopted by municipalities across the country, this is the first formal statement by a body of the federal government. A similar measure has been introduced in the Senate but has not yet been called for a vote.
Executive Order aims to extend “favored nation” pricing to Medicare Part D drugs
On September 13, President Trump signed an Executive Order that links certain Medicare Part D drug prices to the cost of the same drugs in other select countries. The order, which is an extension of another order signed over the summer applying to Part B drugs, requires that the Secretary of Health and Human Services “immediately” test a payment model for Medicare to pay “no more than the most-favored-nation price,” meaning the lowest price paid in other developed countries, for specific “high-cost” prescription drugs and products. The order will only apply to Medicare Part D drugs where “insufficient competition exists.” The order is not without controversy as it faces strong opposition from the pharmaceutical industry, as well as some Republicans who view the action as creating a type of “price control.”
CMS withdraws proposed Medicaid Fiscal Accountability Rule (MFAR)
The Centers for Medicare and Medicaid Services (CMS) announced this week that it is withdrawing its proposed Medicaid Fiscal Accountability Rule (MFAR), which states, hospitals, insurers, patient advocates, and members of Congress on both sides of the aisle had warned could result in major cuts to Medicaid funding. This proposed rule would have restricted how states finance Medicaid programs by, among other provisions, arbitrarily capping supplemental Medicaid payments and placing limitations on intergovernmental transfers.
Duke Health joined efforts urging CMS to reconsider MFAR implementation. While the rule will be removed from the current regulatory agenda, it is unclear whether or not it will be proposed again in the future.
Federal spending and COVID-19 relief
Congress inches toward deal on Continuing Resolution
House Speaker Nancy Pelosi (D-CA), Senate Majority Leader Mitch McConnell (R-KY), and the White House are reportedly close to a deal on a continuing resolution (CR) to keep the government funded beyond the end of the current fiscal year on September 30. The CR is necessary because the House and Senate have yet to reconcile their versions of the 12 annual appropriations bills – and in fact, the Senate has yet to release any public information about its proposed funding levels for FY 2021. Among the final details to work out is the length of the CR, with possibilities ranging from mid-December to early February of next year.
Duke Health Government Relations continues to advocate that funding for medical research be strengthened in FY 2021, including robust funding for the National Institutes of Health (NIH) and Departments of Defense and Veterans Affairs medical research and full funding for the MISSION ZERO grant program. Duke Health and Duke University joined a broader coalition letter led by the Defense Health Research Consortium (DHRC) urging Congress to provide greater certainty for Defense medical research funding outside of a CR.
New life for COVID-19 relief deal? Maybe.
Despite negotiations being all but dead until after the November elections for another round of COVID-19 relief and stimulus, House Speaker Pelosi made a surprise announcement this week that she will not formally recess the House until a deal is reached. The House and Senate were both expected to recess for much of October in advance of the elections, but the move will keep the House in at least open pro forma sessions for the immediate future. While the Senate has yet to respond in kind, the hope is to restart talks that have been bogged down over spending priorities and scope.
Duke Health Government Relations is advocating for at least $26 billion to support the research enterprise and certainty for expanded telehealth policies to continue beyond the public health emergency in any COVID relief package. In conjunction with this effort, Duke Health Government Relations is pleased to see that the “Research Investment to Spark the Economy (RISE) Act” was marked up in the Senate Committee on Commerce, Science, and Transportation. This bill, which was introduced by Senator Thom Tillis (R-NC) among others, would provide $26 billion to support the research enterprise impacted by the pandemic.
Duke Health Government Relations joins Rally for Medical Research
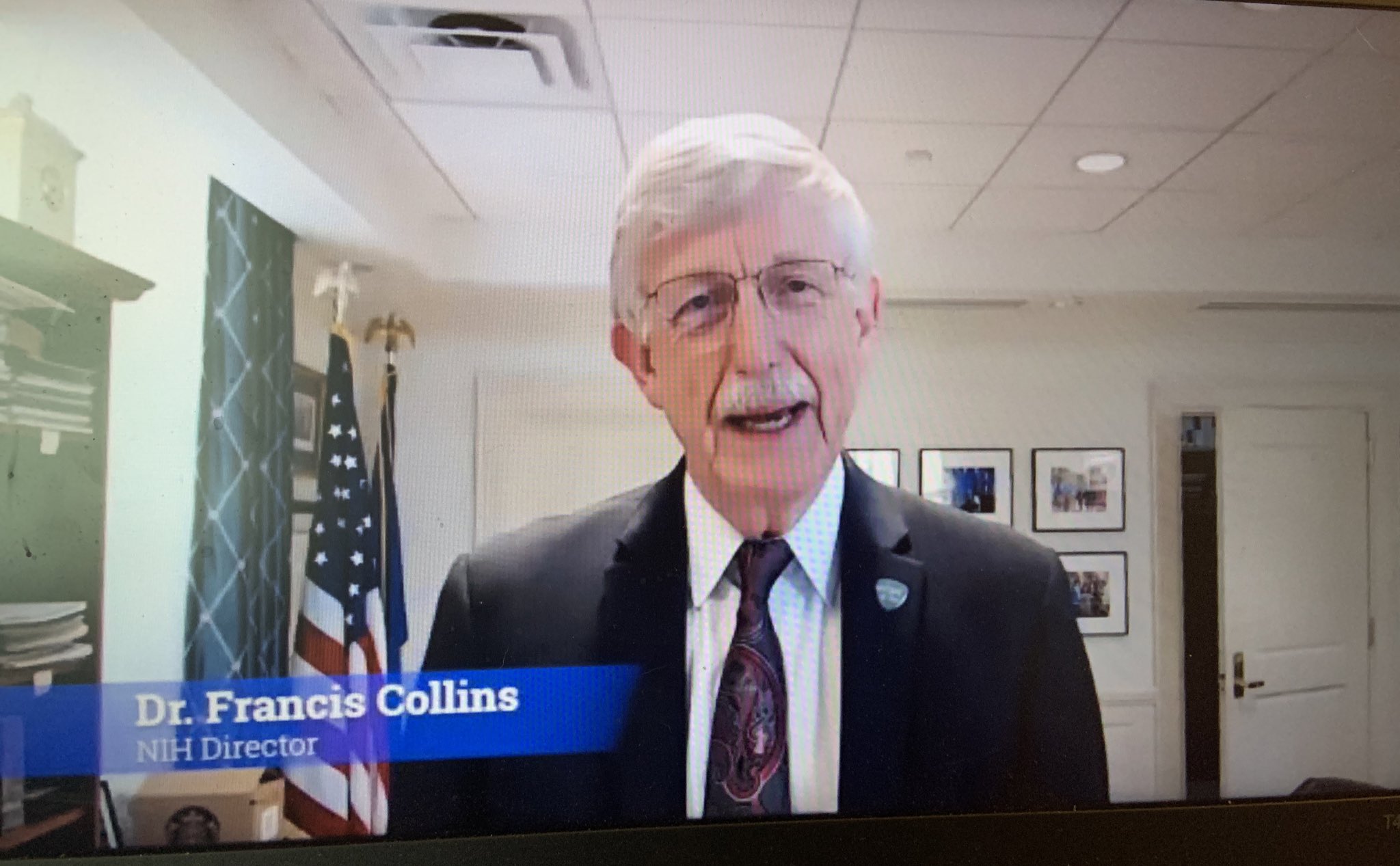
On Thursday, the Duke Health Government Relations team participated (virtually) in the 2020 Rally for Medical Research. Now in its 8th year, the Rally for Medical Research was created as a Capitol Hill engagement event calling on our nation’s policymakers to make funding for the NIH a national priority and to raise awareness about the importance of continued investment in medical research. Our team, along with representatives of over 300 national organizations, met with congressional staff to urge increased funding for NIH for FY 2021, as well as emergency supplemental funding for NIH in any COVID relief and stimulus package.
